AlphaMol discovered potential less-side effect painkiller

Recently, AlphaMol published a research paper titled "Rational Design Biased Compounds against μ - Opioid Receptors" (Doi: 10.1021/acsptsci. 5c00055) in the journal ACS Pharmacology & Translational Science. This study focuses on the µ opioid receptor (MOR), an important analgesic target. Through systematic structure-activity relationship analysis and structural optimization, a new series of opioid receptor agonists has been developed based on existing compounds. Two of these compounds exhibit higher G protein selectivity while maintaining high in vitro activity.

Background
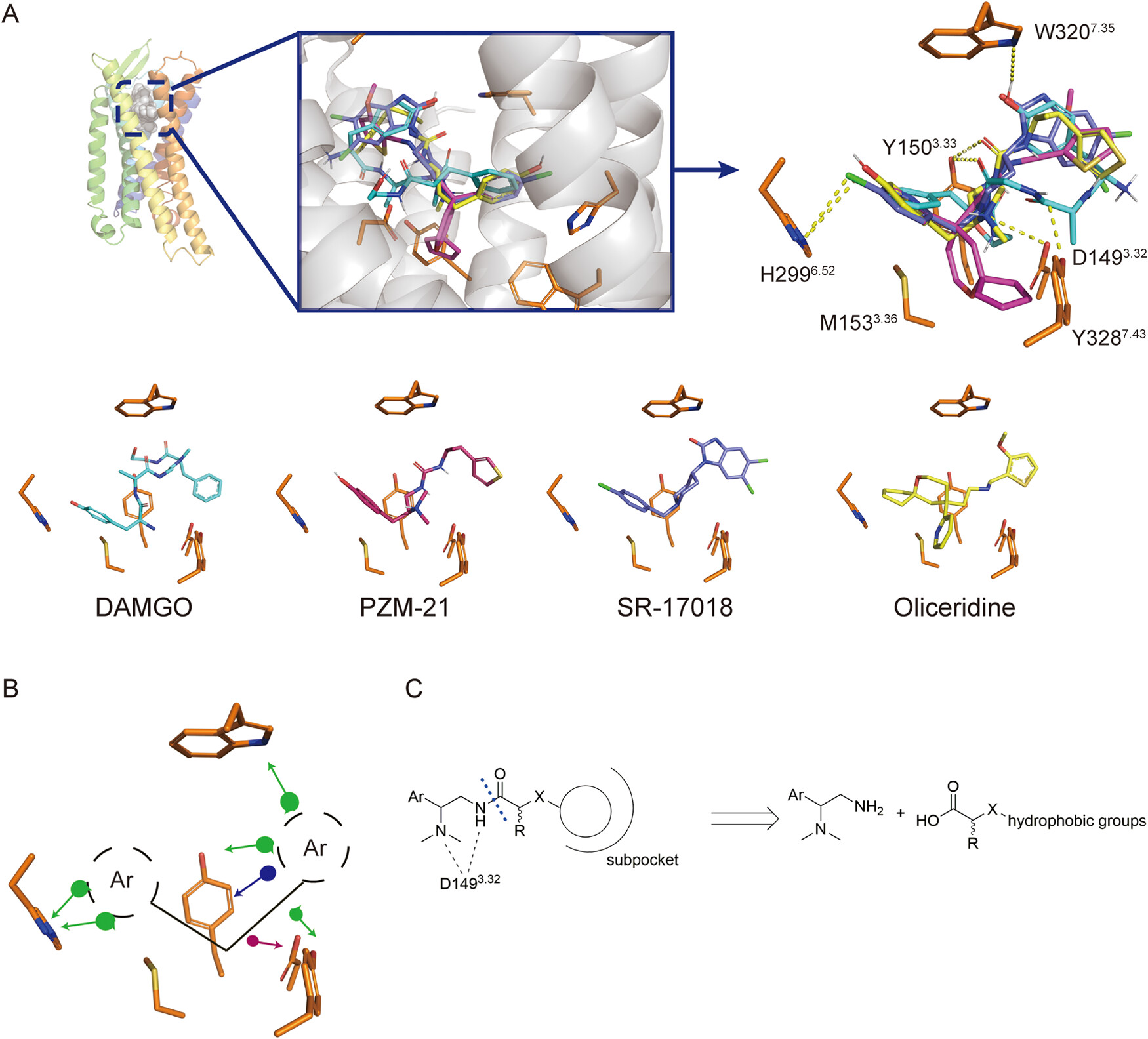
µ opioid receptor (MOR) belongs to class A GPCR family mainly expressed in the central nervous system, which plays a critical role in inhibiting pain transmission. Meanwhile, the activation of MOR can cause adverse side effects, such as respiratory depression, addiction, and constipation, which can directly threaten life in severe cases. The existence of these side effects makes MOR agonists a strictly regulated drug, greatly limiting their clinical application. Therefore, exploring the mechanism of their side effects and developing safer drugs have become research priorities in the field.
Contents
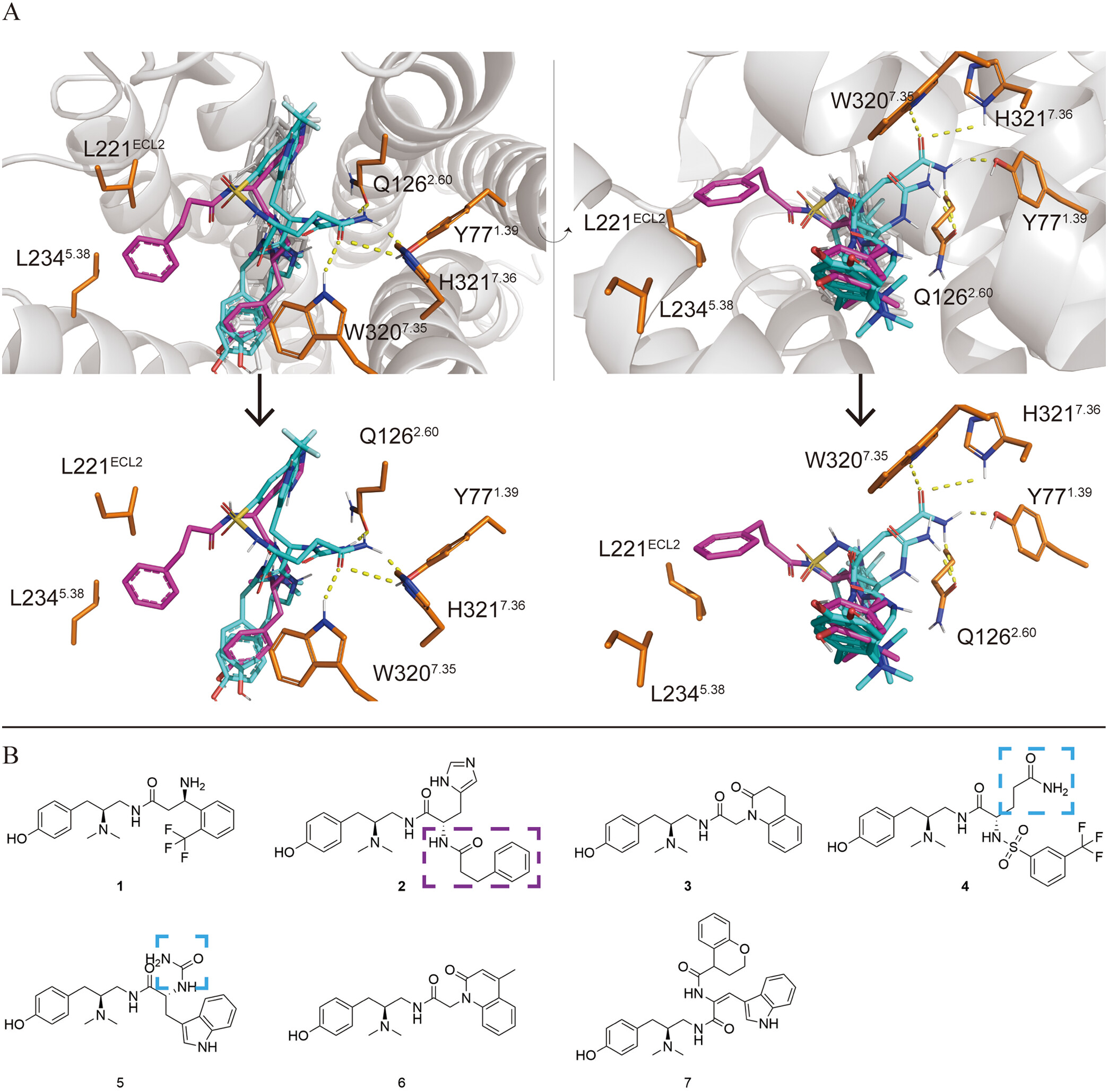
The study aims to develop a novel series of drugs with higher G protein selectivity for investigating the mechanism of MOR side effects. Researchers have rationalized the structure of DAMGO and its derivatives by analyzing their structure-activity relationships. By introducing substituents with different properties into the side chains, it was found that short chain substituents with polar groups at the end can greatly improve the G protein selectivity of the molecule. The researchers discovered two molecules with high in vitro activity (compounds 4 and 5), with compound 4 exhibiting an EC50 of less than 1 nM in the in vitro cAMP experiment. Both compounds also showed higher G protein selectivity, with an EC50 of less than 10 nM for the Gi3 subtype and extremely low activation ability for β-arrestin2 (greater than 10 µ M). Then, the researchers also confirmed the interaction between the two small molecules and MOR through single-point mutation experiments.
Conclusion
The study analyzed the structure-activity relationship of active molecules discovered by previous researchers and their structures with MOR. CADD techniques were applied for rational drug design and structural modification. During the in vitro drug screening experiment, two compounds with high activity were found, and it was confirmed that both compounds had high in vitro activity and G protein selectivity. The results of this study provide two more selective tool molecules and structure-activity relationship verification for the study of side effects of MOR. Based on these two molecules, the effect of activating only specific G protein subtypes on MOR can be studied, thereby expanding the exploration of safer analgesics.